Integrity in response to stress and challenge
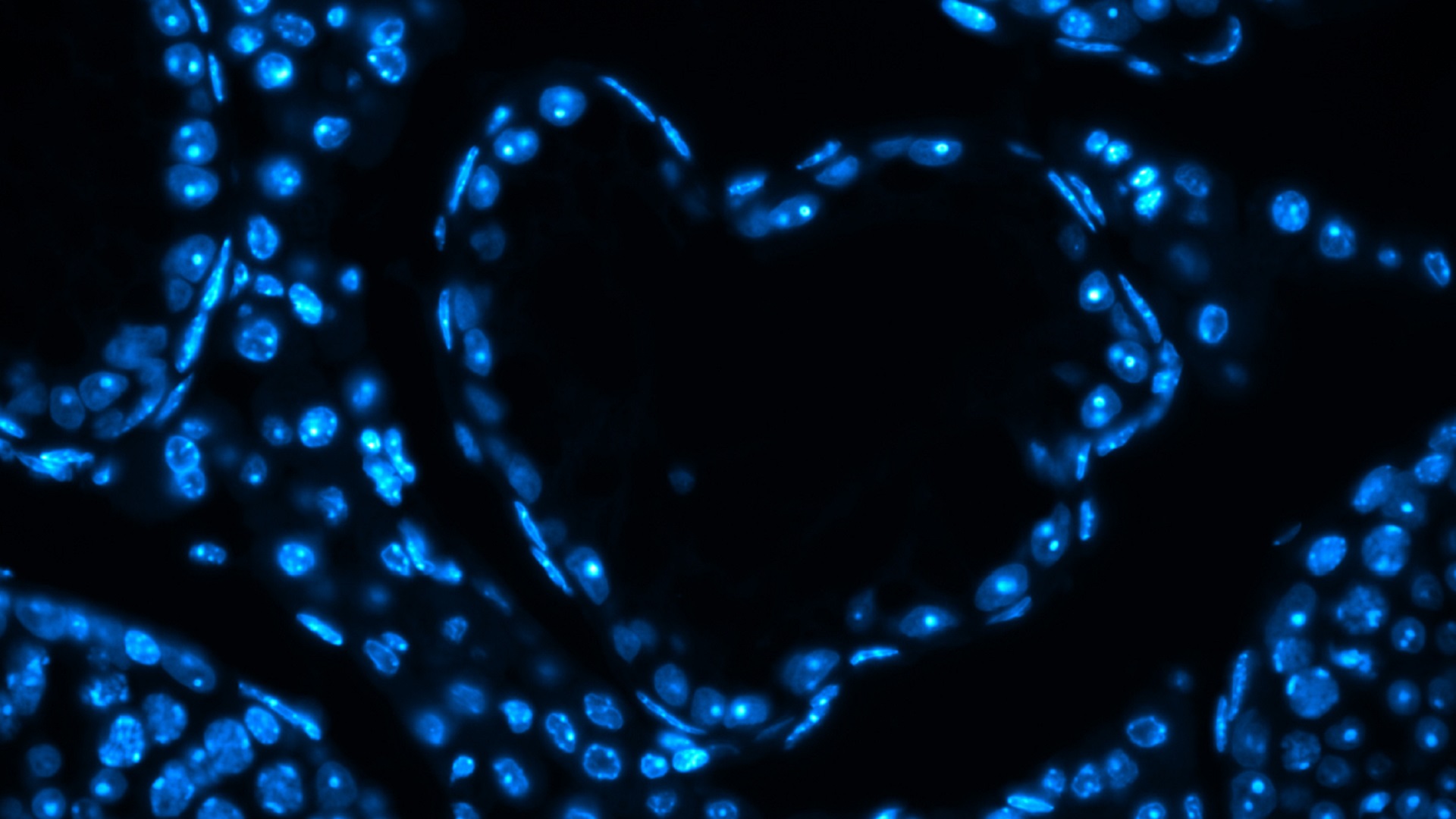
Heart-shaped empty testicular tubule
DAPI staining of testicular tubules from a mouse model of male infertility.
Stress challenges
Most frequent among those challenges, DNA lesions are handled by a complex machinery that ensures correct repair of the nucleic acid molecules. However, beyond genome stability, a key question that we tackle is how the integrity of the epigenome is preserved during DNA damage and repair, and whether the mechanisms and players involved differ according to chromatin context (euchromatin versus heterochromatin) and nuclear organization (compartments). We also explore the consequences of environmental insult at the level of a whole organism, by digging into the interesting concept of epigenetic scars induced by prenatal stress, notably in the context of brain development. Novel mechanisms that explain how stress-responses triggered by prenatal stress protect or alter the epigenome are being deciphered, unravelling both the robustness and vulnerability of the epigenetic layer of information.
Cells and organisms are submitted to unforeseen stressors of external (environmental toxins, drugs) or internal (secreted misfolded cellular proteins) nature. These stressors can challenge cellular integrity by impacting directly on the stability of the DNA and the epigenome or by impairing the function of cell surface receptors important for cellular homeostasis. We study the consequences of internal stressors like amyloid β peptides and the Tau proteins that are secreted as misfolded proteins in the course of neurodegenerative diseases and that have devasting effects on cellular integrity and memory formation. The impact on cellular functions is our primary focus.
From a philosophical viewpoint, identity maintenance and stress response have been conceived in the general framework of robustness. For evolutionary reasons, living systems are robust, both regarding environmental alterations and genetic mutations. This robustness of living systems concerns the functioning of organisms, including stress response, and their development, which involves the concept of canalization as robustness regarding genetic mutations. Response to stress is thus included within a multilayered hierarchical property of organism robustness, which raises two evolutionary issues, namely the causes of its evolution, and its role within evolution. Hence, a future challenge is to situate the Labex’s findings on stress response within a general theory of robustness that would take into account its hierarchical structure and its dual evolutionary meaning.
Mechanical cues and signaling
Control of cell and tissue identity has long been shown to be triggered by morphogens and downstream signaling pathway. However, recent accumulating experimental evidence led to the recognition that mechanical properties of tissues can control a variety of cell functions including cell stemness, cell proliferation and cell death. Physical properties of the tissue environment appear as a key regulator of its maintenance and integrity. Along this line, we focus on the impact of mechanosensing and mechanotransduction pathways on cell assemblies. This may rely on properties emerging at the multicellular level, associated to the physical properties of the environment, the position of the cells relative to each other and to the coexistence and complex interaction of multiple cell types over space and time. Adult epithelia, for example, are in a constant dynamic equilibrium of cell proliferation, migration, differentiation and cell death. Such homeostasis process is highly important as it is the key of functional tissue renewal and it maintains the epithelial integrity. A revolutionary breakthrough in the development of organoids in vitro from isolated adult stem cells has been achieved in the past decade. As another example, the development of the skeletal muscle cells implicates a tight coordination of adhesion, contraction and interaction with their environment. In the case of motile and contractile cells such as muscle cells, the coupling to various (i.e. soft or stiff) substrates can control the fusion processes and thus muscle formation and repair.
In this context, forces, cell-cell interactions and physical changes in micro-environments may modulate the transduction of mechanical signals across the cell membrane and hence modify the fusogenic properties of primary myoblasts and myofibers. The rapid development of micro/nano-engineering techniques and functional biomaterials have empowered biologists and bioengineers to precisely control aspects of the in vitro cell microenvironments. How cues from tissue geometries and environment mechanical properties may control the maintenance of stemness and cellular identities remains a puzzling issue to understand tissue homeostasis. These include to understand how the dynamics and organization of multicellular systems, as well as how stem cell proliferation and differentiation impact self-renewal equilibrium, and ultimately cell proliferation, death and extrusion. Here, we focus on physical cellular sensing perceived by cell-cell adhesion complexes that can propagate over multiple cells. It then leads to an internal rearrangement at the inner cell membrane together with active forces exerted by the actomyosin cytoskeleton that propagate from the cytoplasm to the nucleus but also. Recent studies have shown that various transcription factors including YAP, β-catenin can shuttle between the cytoplasm and the nucleus in response to mechanosensing in cellular monolayers. We study the interplay between mechanical constrains and molecular mechanisms implicating chromatin dynamics, gene localization and expression and nuclear transport across nuclear pores to understand tissue-scale homeostasis. The perception of mechanical signals from molecular to multicellular scales over various time-scales have strong implications to define single-cell identity within a population.
À lire aussi
Closing Conference 2024 – Interdisciplinary perspectives on identity
The Labex Who Am I? invites you to its closing conference, “Interdisciplinary Perspectives on Identity: from Single Cells to Organism and Beyond”, which will take place on November 27-29 in Paris. The Labex Who Am I? final conference will take place on November 27, 28...

Pint of Science festival 2024
Once again this year, the Labex Who Am I? is partnering with the Faculty of Sciences of the Université Paris Cité, the Genetics and Epigenetics New Education (G.E.N.E.) Graduate School, and the Major Research and Innovation Domain BioConvergence for Health...
Visiting Professors programme: Matthias Peter
As part of its Visiting Professors programme, the Labex Who Am I? is delighted to welcome professor Matthias Peter, professor of Biochemistry and group leader at ETH Zurich, Switzerland. © Peter group, ETH Zurich Matthias Peter...

Rhythms and resonances: coming into contact with otherness
The Labex Who Am I? co-funds the “Rhythms and resonances: coming into contact with otherness” workshop. © Gerd Altmann from Pixabay Interdisciplinary doctoral workshopThe “Rhythms and resonances: coming into contact with otherness”...
