Malaria: Blocking Infected Red Blood Cells to Halt Transmission
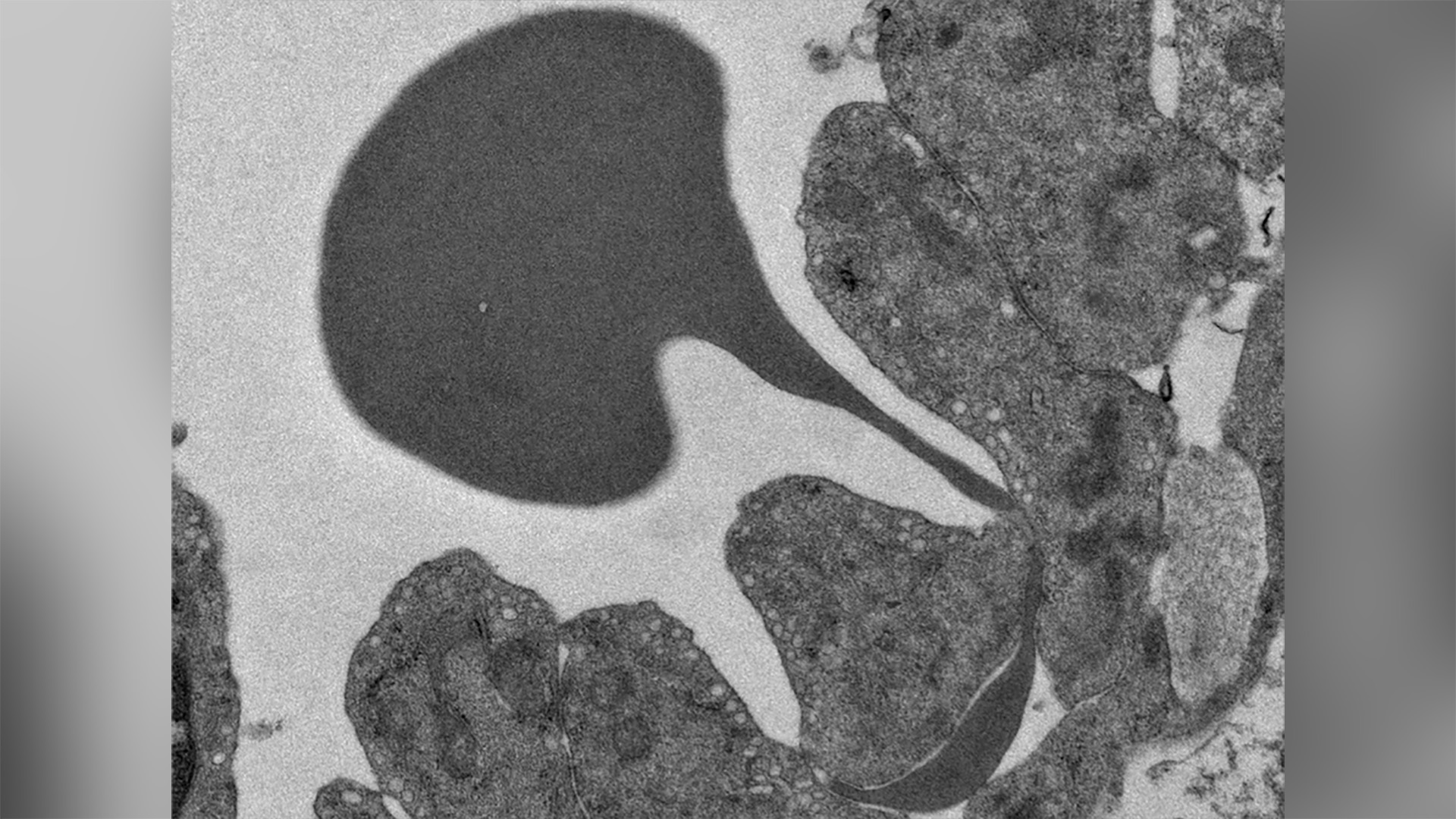
© Abdoulaye Sissoko, Sonia Gorgeault, Philippe Roingeard, Pierre Buffet
Red (dark grey) globule passing through the wall of a sinus of the spleen
In a recent study, Professor Pierre Buffet’s team (UMRS 1134, Université Paris Cité – Inserm), was studying the proportions of red blood cells infected by the malaria parasite present in the blood and spleen. The study explained the mechanisms of blood filtering by the spleen and identified 2 drugs likely to increase the efficiency of this filtering. The infected red blood cells would then be held in the spleen to be destroyed and eliminated, thus stopping the transmission of the disease. These results are published in the journal Nature Communications.
Malaria is a potentially fatal infectious disease caused by several species of microscopic parasites belonging to the genus Plasmodium. Transmitted to humans by an infected mosquito bite, the parasite settles in the liver, then multiplies in the red blood cells, and bursts them. It can then spread and infect more and more red blood cells.
Moving from Infection to Disease
The malaria parasite evolves from an asexual form responsible for the infection to a sexual form, called a gametocyte, responsible for the transmission of the infection.
The asexual form multiplies for a few days, making the patient become ill. Once treated, the asexual forms of the parasite in the blood disappear and so do the symptoms. However, the sexual forms of the parasite (derived from the asexual forms) remain in the blood for 2 to 3 weeks, causing the patient to become contagious, and therefore a transmitter of the infection.
The spleen, a Built-in Filter
In the body, the spleen is the organ simultaneously responsible for generating an immune response to germs in the blood and for filtering the blood to destroy and eliminate abnormal, old or infected red blood cells. In a previous study, researchers at Institut Pasteur (Innocent Safeukui, Peter David, Geneviève Milon and Pierre Buffet, amongst others) had demonstrated that, in infected patients, while half of the infected red blood cells are contained in the spleen to be destroyed, the other half return to the bloodstream where they carry out harmful effects and continue the infection mechanisms of healthy red blood cells. Based on this observation, the team sought to understand why the spleen’s role as a filter is not effective on all infected red blood cells.
The spleen is a unique organ in which blood exits the vessels to come into contact with macrophages responsible for “analysing” the red blood cells to destroy those that have become abnormal, too old or infected. Blood enters the spleen through an artery and leaves through a vein. In the spleen, the red blood cells are in direct contact with the macrophages, outside the usual circulatory network, and then pass through the walls of small veins (the sinuses), undergoing major deformation, to return to the general bloodstream. Only normal red blood cells can cross this mechanical barrier and re-enter the bloodstream.
In malaria patients, infected red blood cells enter the spleen and some do not leave. The researchers therefore hypothesized that infected red blood cells, which have a normal membrane surface, may simply have difficulty in deforming to get past the sinus wall and back into the bloodstream. They then studied the physical properties of infected red blood cells and showed that they are more rigid than healthy red blood cells. This means that infected red blood cells are less able to bend to pass through the spleen filter.
The research team then wanted to pursue their investigations by relying on this property of “acquired rigidity” of red blood cells during infection. Their assumption: if a molecule is able to increase the rigidity of all malaria-infected red blood cells sufficiently, then the spleen will be able to hold back all infected red blood cells so that macrophages can destroy them, thus putting an end to the transmission of the infection.
Treating the Population to Stop the Disease
In order to do this, the research team, with Julien Duez and Mario Carucci as main players, in tight collaboration with the malaria group lead by Javier Gamo and Laura Sanz-Alonso at GSK Tres Cantos, tested the activity of more than 13,000 drug molecules on gametocyte-infected red blood cells, all of which have already been evaluated in Phase 1 clinical trials in humans. The upside is that for these molecules, researchers have a wealth of information on tolerance as well as their administration method.
The screening of the 13,555 molecules, the first part of the study, made it possible to identify 112 drugs that are active and therefore make infected red blood cells rigid at high doses. The second step was to evaluate for these 112 drugs, the optimal dose to be administered to reach the degree of stiffness of the infected red blood cells sufficient to contain them in the spleen. The team then analysed the complete pharmacological data for these drugs and searched for which the effective dose could be achievable in the blood of those treated with a single oral dose. At the end of the study, only 2 molecules were selected: cipargamine and TD-6450.
Further studies must now be conducted in humans to ensure that these two drugs, administered orally for a very short period of time, do indeed stop the circulation of gametocyte-infected red blood cells in all patients and that, as a consequence, transmission is also halted.
Source
Safe drugs with high potential to block malaria transmission revealed by a spleen mimetic screening
DOI : https://doi.org/10.1038/s41467-023-37359-2
Contacts
presse@u-paris.fr
Read more
![[Circle U.] Fast Forward Open Science](https://u-paris.fr/wp-content/uploads/2025/09/CC.-OPEN-ACCESS-WEEK-1080x675.png)
[Circle U.] Fast Forward Open Science
On the 22nd of October from 1:30 to 4:30 pm, Circle U. will host the online event ‘Fast forward Open Science’, led by Université Paris Cité, as part of the International Open Access Week 2025. Experts in open access publishing, research data management, open source...

No relationship between Université Paris Cité and the private company operating under the name City University of Paris
Université Paris Cité discovered that a private higher education establishment initially called Intelligest has recently changed its company name for ‘City University of Paris’. Université Paris Cité points out that it has no links whatsoever (association,...

Results of the 2025 Call for Projects with the University of Toronto
The 2025 call for projects between Université Paris Cité and the University of Toronto met with great enthusiasm within the scientific communities of both institutions. Eighteen proposals were submitted by pairs of researchers; five projects were selected at the end...

Call for projects 2025 UPCité – King’s College London
The call for projects between Université Paris Cité (UPCité) and our privileged partner King's College London (KCL), has been launched this friday, May 9th 2025. The objectives Université Paris Cité and King's College London are offering offering a seed funding for...
