Nearly 20% of cancers are associated with microbial infections. While the mechanisms involved are fairly well-known for viruses and bacteria, the role played by parasites remains a mystery. A new study led by Prof. Jonathan Weitzman from the Epigenetics and Cellular Fate unit, focused on Theileria annulata, a tick-borne parasite that causes a cancer-like disease in cows, killing the animal within days. The study, published in Nature Communications, describes a modification of the histone proteins that allows the parasite to regulate the expression of its genes, a mechanism that has never been reported in mammals.
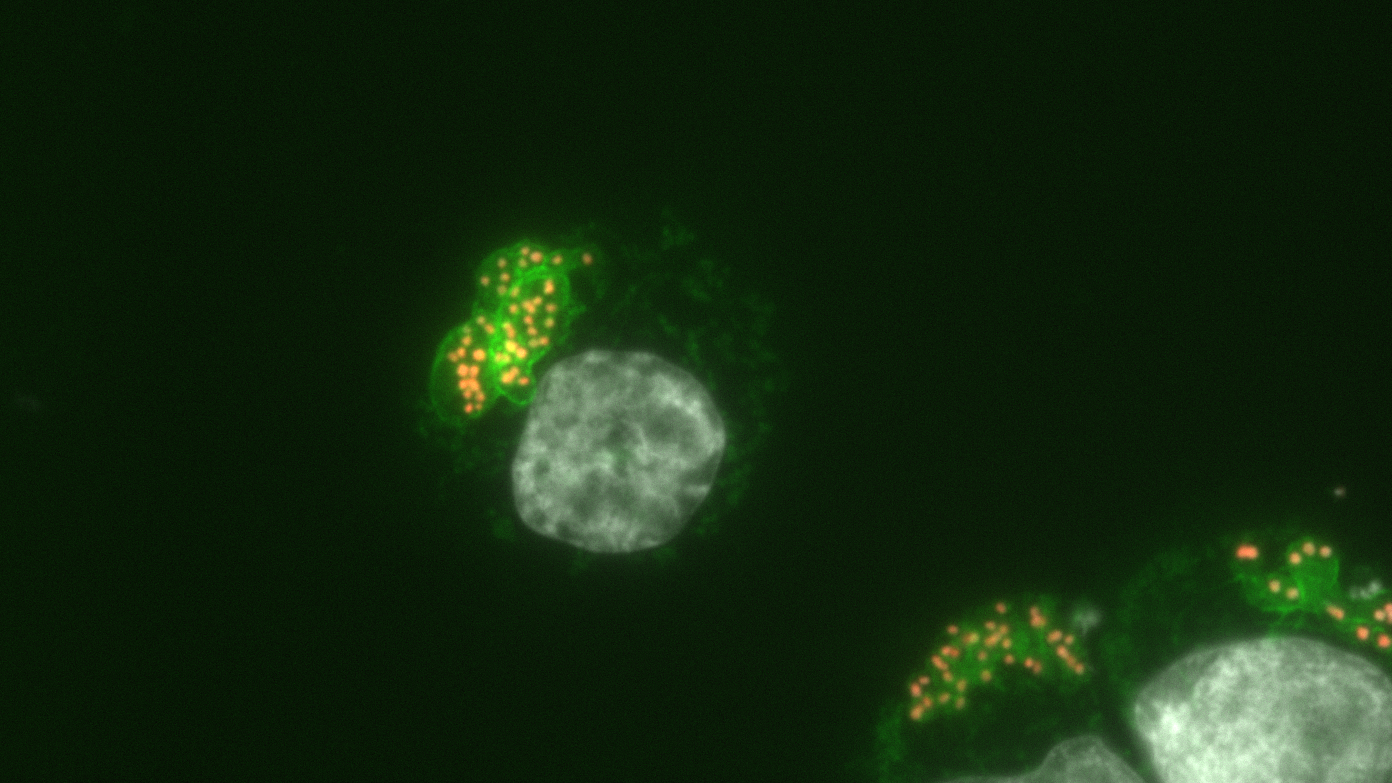
Bovine macrophages infected with Theileria annulata.
Green fluorescence highlights the surface of the parasite.
Parasite nuclei are indicated by the new epigenetic mark (called H3K18me, in orange), but the bovine host cell nucleus is devoid of this mark (in grey).
© Laboratoire Épigénétique et Destin Cellulaire
Single-cell parasites may be small organisms, but they are responsible for some of the world’s most severe diseases in both humans and animals. A better understanding of how these parasites work and what makes them unique is a major challenge to developing effective drugs and vaccines.
Theileria annulata, a single-cell parasite, causes a fatal disease in cattle in Africa and Asia, resulting in a significant socio-economic impact. Although a drug exists to treat this parasite, the treatment becomes ineffective when the parasite undergoes a genetic mutation that causes drug-resistance. It is therefore important to understand the particular behavior of Theileria parasites in order to identify new therapeutic strategies.
While using standard molecular genomic and biochemical tools, the study conducted by Prof. Jonathan Weitzman’s team deviates from the classical experimental genetic models and focuses on the epigenetic mechanisms involved in the infection by this parasite.
Theileria annulata: an unforeseen threat
Transmitted to cows by ticks, this parasite infects white blood cells, multiplying within the host cells and transforming them into “immortalized” cancer-like cells. Studying this disease in cattle can provide insights into cancer, a disease that involves both genetic and epigenetic [1] mechanisms.
In an earlier study, published in 2015 in Nature, Prof. Jonathan Weitzman’s team described the mechanism by which Theileria annulata makes the host cells, white blood cells, “immortal.” The team discovered a new parasite protein that is secreted into the host cell and alters the cell identity to drive replicative anarchy, making them “immortal” like human cancer cells. This uncontrolled cell division allows the parasites to spread to the other organs, causing the death of the animal.
In a new study, published on May 28th, 2021 in Nature Communications, the team discovered a new epigenetic mechanism by which this parasite is able to regulate the expression of its own genes (turning the genes), in a surprising way.
[1] While genetics is specifically concerned with genes, their DNA sequence and potential mutations, epigenetics explores the factors that influence the use or non-use of genes by the cell and their behaviour. Unlike mutations, which can affect the DNA sequence of one or more genes, epigenetic modifications are reversible.
A mechanism never before described in mammals
Contrary to bacteria and viruses, Theileria annulata is a eukaryotic parasite. Like mammals, its cells contain a nucleus where the DNA and its genes are located.
The team of geneticists and biochemists showed that the Theileria parasite has a unique epigenetic feature that regulates its genes. They also discovered a new protein (they named TaSETup1) that controls the parasite genes using this epigenetic mechanism. This mechanism, never before described in mammals, is essential for the life cycle of the parasite. The researchers are now developing drugs to target the TaSETup1 protein and its epigenetic target.
“It’s remarkable that we can still learn so much about how genes are turned on and off by studying a tiny single-cell organism”, marvels Professor Jonathan Weitzman, adding that this is the first time the epigenome of these parasites has been studied in such molecular detail.
This discovery suggests that drugs capable of blocking the secreted proteins or the TaSETup1 epigenetic enzyme could block the parasite life cycle and prevent it from spreading, first to ticks and subsequently to cattle. This study offers unprecedented insights into how this clever parasite controls the expression of its genes and offers new hints about how infections can cause cancer.
This study was financed with the support of the ANR and the Labex Who am I ?
References
Dynamic methylation of histone H3K18 in differentiating Theileria parasites. Cheeseman K, Jannot G, Lourenço N, Villares M, Berthelet J, Calegari-Silva T, Hamroune J, Letourneur F, Rodrigues-Lima F, Weitzman JB.
DOI : https://doi.org/10.1038/s41467-021-23477-2
Theileria parasites secrete a prolyl isomerase to maintain host leukocyte transformation. Marsolier J, Perichon M, DeBarry JD, Villoutreix BO, Chluba J, Lopez T, Garrido C, Zhou XZ, Lu K, Fritsch L, Ait-Si-Ali S, Mhadhbi M, Medjkane S, Weitzman JB.
DOI : https://doi.org/10.1038/nature14044
Professeur Jonathan Weitzmann
Laboratoire Épigénétique et Destin cellulaire
UMR 7216 CNRS – Université Paris Cité
Membre senior de l’Institut Universitaire de France
Read more

The Indian Institute of Science Education and Research in Pune and the Indian Institute of Technology in Bombay: UPCité’s first partners in India.
Antoine Kouchner, Vice-President of Strategy and International Relations at Université Paris Cité, travelled to India for the official visit of the President, Emmanuel Macron, to the High-Level Scientific and Academic Meetings (RUSH). In this context, UPCité...

International Day of Women and Girls in Science: celebrating the women who push research forward
February 11 was the International Day of Women and Girls in Science. On this day, Université Paris Cité reaffirms its commitment to the equality between men and women and celebrates the journey of the women who advance research. Between celebrating our heritage and...

INC Day 2025: an international scientific day dedicated to neuroscience
The Neuroscience and Cognition Institute of Université Paris Cité (INC) organized a new edition of the INC Day, focused on neurodevelopmental trajectories. A key partner of the event, the Graduate School Neuroscience invited its first year and second year master...

Abraha and Pierre: A Friendship to Preserve the People’s Memory of War
In Paris, two historians’ paths crossed. One had just arrived from Ethiopia, carrying notebooks filled with daily observations written during the war in Tigray. The other, based in France, is a specialist of Ethiopian modern history. From this encounter, a partnership...
