Targeted Treatments: Biomanufacturing Therapeutic Delivery Vectors
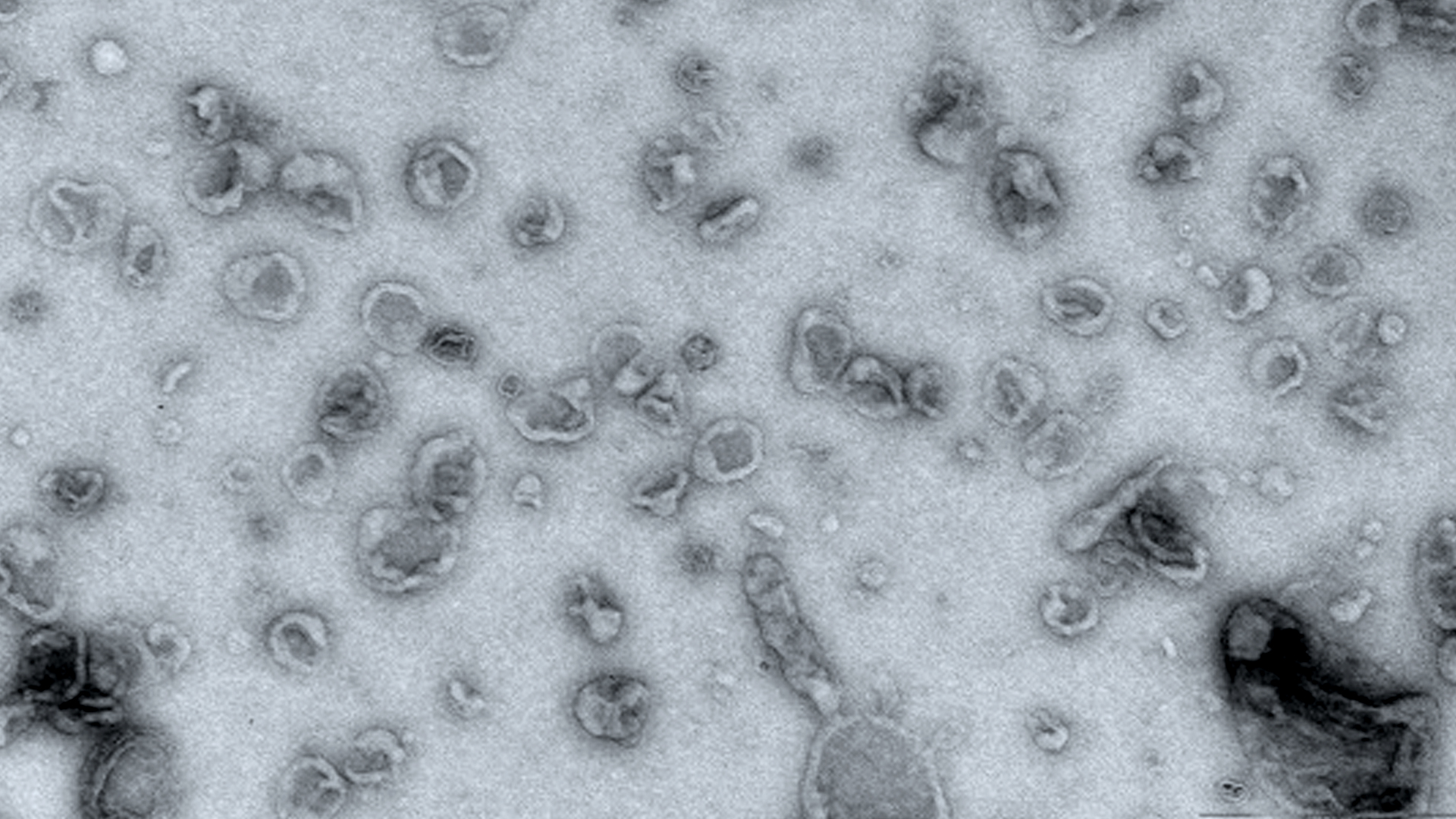
Extracellular vesicles observed by electron microscopy.
In two recent publications, Grégory Lavieu, chair of Excellence IdEx-Université Paris Cité, along with researchers, Shéryl Bui and Julia Dancourt, (UMR7057 CNRS-Université Paris Cité-Inserm U1316), developed cell biomanufacturing processes capable of producing extracellular vesicles designed to carry and deliver therapeutics into recipient cells, notably cancer cells. This new method does not involve any viral component, which is usually used in the manufacture of vectors, and thus avoids manufacturing constraints and health risks. This discovery may open promising doors in the manufacture of virus-free and EV-based delivery systems that could be integrated into cell and gene therapy applications.
Known for around 30 years, extracellular vesicles are small vesicular sacs produced by cells and capable of transporting a number of molecules outside the cell. These extracellular vesicles (EVs) have long been considered as simple transporters secreted by all kind of cells/tissues and circulate in bodily fluids.
In previous studies, the research team had demonstrated that EVs were used to transport different biomaterials (proteins, nucleic acids…) from a donor cell to a recipient cell. They explained and quantified the process of vesicular content release, highlighting the potential that these EVs represented for their intended use as drug delivery vectors.
Pursuing their investigations, the research team led by G. Lavieu, characterises uptake and cargo delivery mechanisms step by step at the cellular and molecular level.
Searching to use the natural properties of donor cells, the team wondered if it was possible to biofabricate donor cells producing EVs with specific properties. With the goal of creating drug delivery vectors for targeted treatments, the researchers thus proceeded in several stages.
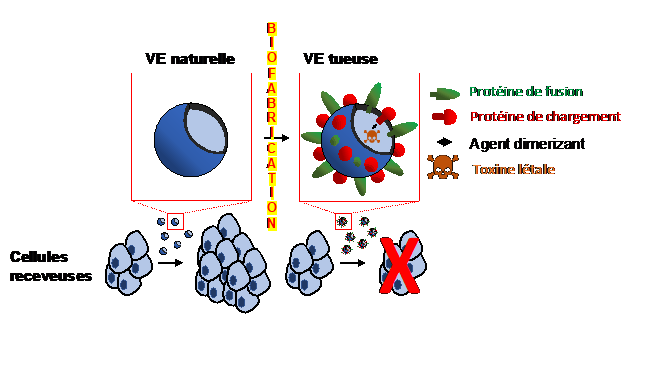
The first step was to use genetic tools to biofabricate killer vesicles loaded with a lethal toxin and decorated with non-viral fusion proteins to enhance the delivery of the toxin into the acceptor cells. This suggests that eventually these killer vesicles could expand the existing cancer therapeutics arsenal for tumor ablation.
The researchers then developed a technique to load this toxin (or any other protein of interest) into these extracellular vesicles in a reversible manner. At the same time, the EVs were equipped with a fusogenic molecule of human origin, enabling to increase the fusion of the membranes and thus the discharge of the vesicular contents into the acceptor cells. Typically the fusogenic agent is viral in nature, which requires numerous production limitations and health risks. Once these fusogenic EVs were produced by the donor cells and loaded with the lethal toxin, the researchers isolated the EVs and loaded them on acceptor cells in culture. The EVs then fused with the acceptor cells and released their vesicular (toxin) contents inside these cells, causing the latter to die.
These breakthroughs and the development of new biomanufacturing methods have led to a significant progress in the development of target treatments, notably for cancer. The method is versatile, enables the uptake of a broad range of therapeutic proteins, with the potential to complement the existing arsenal of cell and gene therapies.
Sources
1– Efficient cell death mediated by bioengineered killer extracellular vesicles
Julia Dancourt, Ester Piovesana & Gregory Lavieu
https://www.nature.com/articles/s41598-023-28306-8
2- Virus-Free Method to Control and Enhance Extracellular Vesicle Cargo Loading and Delivery
Sheryl Bui, Julia Dancourt, Gregory Lavieu
Biofabrication of killer vesicles loaded with a lethal toxin and decorated with non-viral fusion proteins to enhance the delivery of the toxin into the recipient cells leads to the elimination of these same cells. Eventually, these killer vesicles could complete the therapeutic arsenal for tumor ablation.
Read more

Call for applications: SMARTS-UP Graduate Schools incoming mobility scholarships for master’s students
The SMARTS-UP Graduate Schools programme at Université Paris Cité aims to promote the internationalisation of Master's programmes and facilitate the admission of top international students, creating a pool of excellence for the recruitment of future PhD candidates....
read more![[SMARTS-UP Graduate Schools call for applications] Outgoing mobility scholarships for master’s students](https://u-paris.fr/wp-content/uploads/2021/04/SmartUP_1920-1080x675.jpg)
[SMARTS-UP Graduate Schools call for applications] Outgoing mobility scholarships for master’s students
The SMARTS-UP Graduate Schools programme at Université Paris Cité aims to promote the internationalisation of Master's programmes and facilitate the integration of top students into international organisations, creating a pool of excellence for the recruitment of...
read more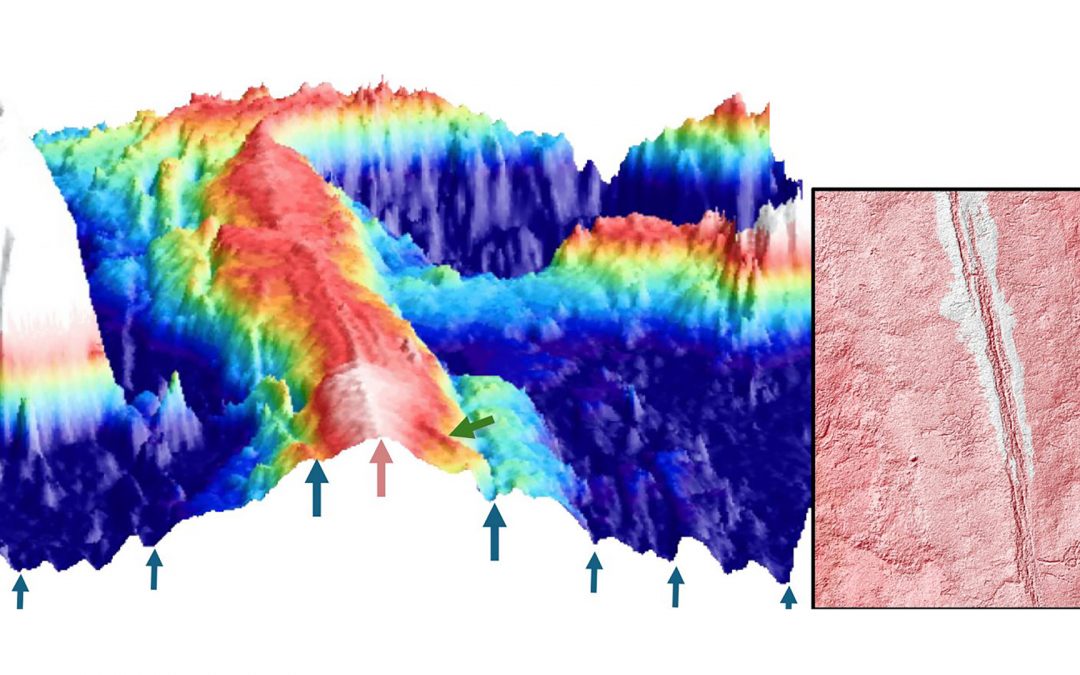
Evidence of magmatically induced faults at the East Pacific Rise
By comparison of ultra-high-resolution 3-D seismic imagery and bathymetry data collected at the East Pacific Rise (EPR) 9º50'N, researchers reveal the existence of magmatic induced faults near the ridge axis.
read more
Convergences: A Strategic Alliance paving the Future of Biology-Health Research
A key milestone for the partnership between Institut Pasteur and Université Paris Cité, the first “Convergences” scientific seminar took place on June 17th at the university’s head office at Odéon.
read more