The Lavieu Lab
In order to help strengthen the international scientific positioning and attractiveness of Université Paris Cité, an action “IdEX Chairs” has been launched. This call for proposals aims to promote the hosting of researchers from outside the institution whose profile corresponds to internationally renowned seniors or juniors with very high potential.
Already 7 laureates in the last two years, including Grégory Lavieu who joined Université Paris Cité on the research site of the Saint-Germain-des-Prés campus to set up a team dedicated to intra- and extracellular membrane transport and thus strengthen the Cell Biology cluster.
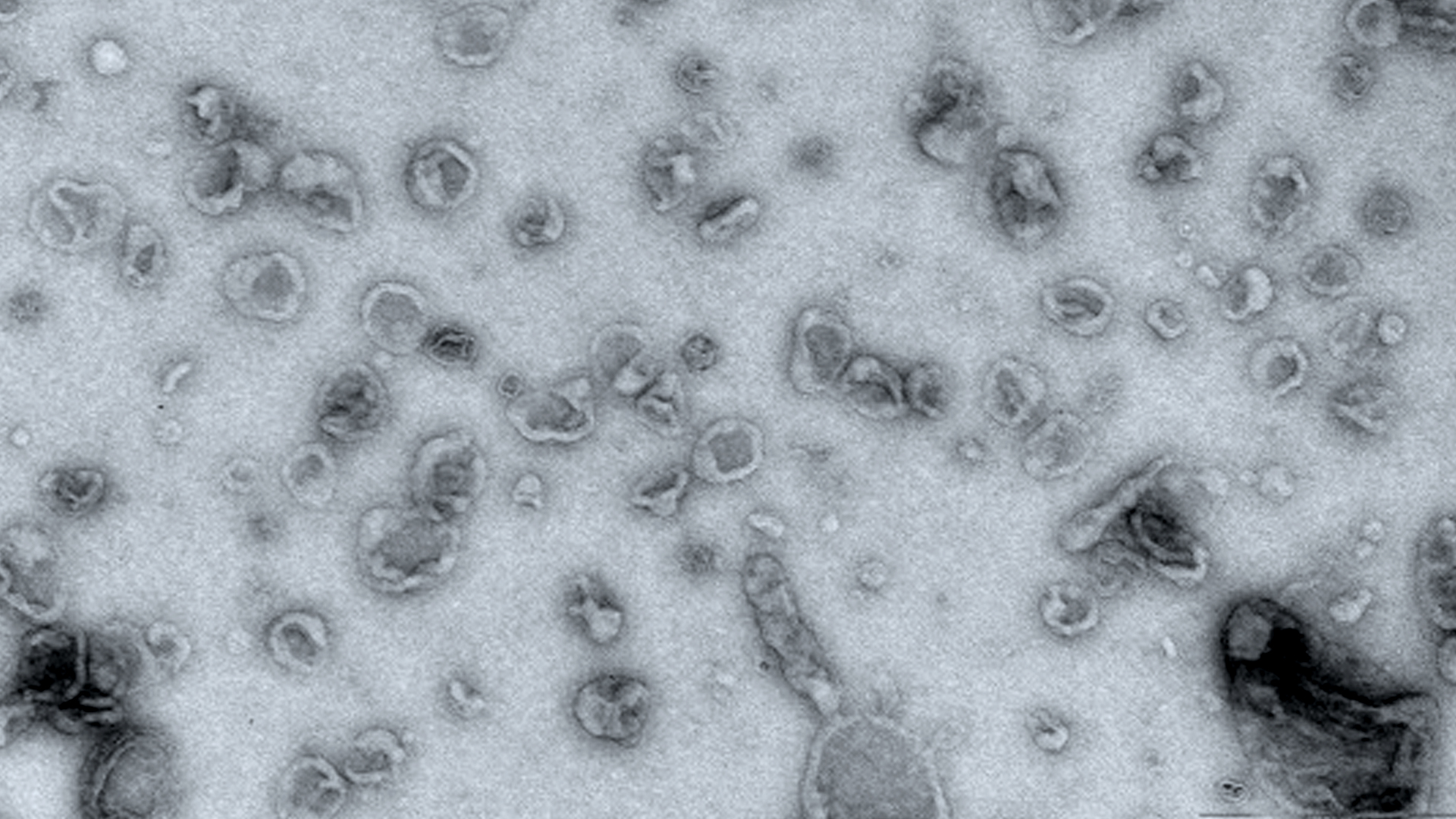
Electron micrograph showing extracellullar vesicles.
Director
 Gregory Lavieu, PhD (PI), INSERM Research Director.
Gregory Lavieu, PhD (PI), INSERM Research Director.
Lavieu is a cell biologist/biochemist expert in membrane trafficking and leader of the “Intra and extracellular membrane trafficking “ team hosted by Université Paris Cité, , INSERM U1334, CNRS UMR8175, Department of Biomedical and Fundamental Sciences, located in the Campus Saints-Germain-des-Prés, Paris.
G. Lavieu and his research group aim at characterizing the intercellular transport mediated by extracellular vesicles (exosomes), at both cellular and molecular levels. Our goal is to identify the core machinery controlling this process.
Contact
Gregory Lavieu, PhD
Chaire IdEX Université Paris Cité
INSERM ERL U1316 Membranes Dynamics In and Outside the Cell
INSERM, UMR7057/MSC, Bureau P335B
45 rue des Saints-Pères,
75006, Paris, France
Phone : +33 176534267
Gregory.lavieu@inserm.fr
Research:
Extracellular Vesicles (EVs), including Exosomes, are now recognized as vectors of intercellular communication capable of transferring nucleotides, lipids, and proteins from donor to acceptor cells. EV-mediated communication has been associated with many physiological and pathophysiological functions, including cancer, immune responses, cardiovascular diseases, lipid homeostasis, tissues regeneration and stem cell-based therapy.
EVs are being recognized as vectors of major importance for physiology in general, and appears as promising candidates for translational applications such as targeted therapeutics delivery.
However, the mechanisms responsible for EV delivery within the acceptor cells remain unknown at both the cellular and the molecular levels. How do vesicles enter cells? Is it receptor-dependent? How do vesicles deliver their contents within the cytosol of the acceptor cells? Does it require membrane fusion? If yes, what is the nature of the target membrane and the fusion machinery? Those basic questions are not yet answered.
This is not satisfying, especially considering how much we know about the cellular and molecular mechanisms that regulate the delivery of viruses or the transport of intracellular vesicles, which both share several physico-chemical properties with EVs.
It is therefore of high priority to close these gaps, especially when considering the high translational impact of EVs.
We developed cell-free and cell-based assays to accurately assess in a qualitative and quantitative manner the EV delivery process, especially the EV content release. Our results suggest that EV content delivery occurs within the endo-lysosomal compartment, through a process that is pH- and protein-dependent and that seems to involve membrane fusion. We are now aiming at identifying the core machinery that controls this process, to later engineer EV-mimetics designed for targeted therapeutics delivery.
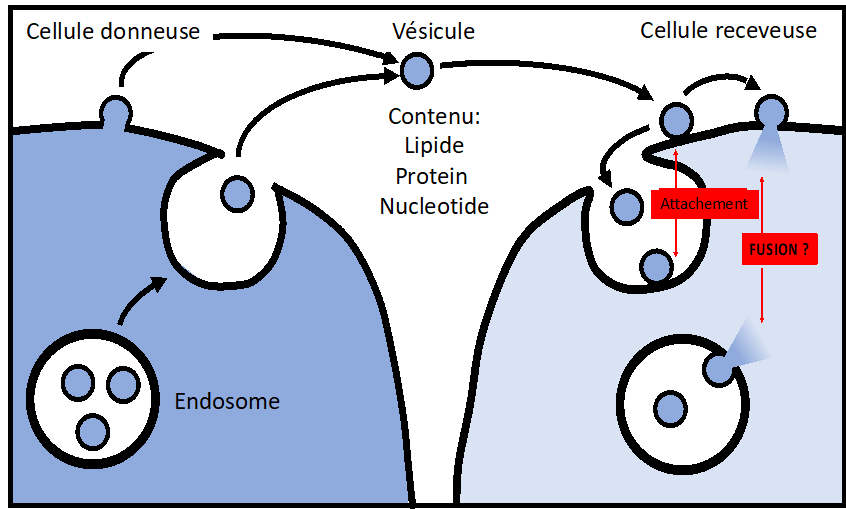
Routes for EV Uptake and Content Delivery
Publications
Selected relevant publications:
- Tognoli ML, Dancourt J, Bonsergent E, Palmulli R, de Jong OG, Van Niel G, Rubinstein E, Vader P, Lavieu G. Lack of involvement of CD63 and CD9 tetraspanins in the extracellular vesicle content delivery process. Commun Biol. 2023 May 17;6(1):532. doi: 10.1038/s42003-023-04911-1. PMID: 37198427; PMCID: PMC10192366.
- Bui S, Dancourt J, Lavieu G. Virus-Free Method to Control and Enhance Extracellular Vesicle Cargo Loading and Delivery. ACS Appl Bio Mater. 2023 Mar 20;6(3):1081-1091. doi: 10.1021/acsabm.2c00955. Epub 2023 Feb 13. PMID: 36781171; PMCID: PMC10031566.
- Dancourt, J., Piovesana, E. & Lavieu, G. Efficient cell death mediated by bioengineered killer extracellular vesicles. Sci Rep 13, 1086 (2023). https://doi.org/10.1038/s41598-023-28306-8
- Bonsergent E, Grisard E, Buchrieser J, Schwartz O, Théry C, Lavieu G. Quantitative characterization of extracellular vesicle uptake and content delivery within mammalian cells. Nat Commun. 2021 Mar 25;12(1):1864. doi: 10.1038/s41467-021-22126-y. PMID: 33767144; PMCID: PMC7994380.
- Bonsergent E, Lavieu G. Content release of extracellular vesicles in a cell-free extract. FEBS Lett. 2019 doi: 10.1002/1873-3468.13472.
- Spotlight on early-career researchers: an interview with Gregory Lavieu. Commun Biol. 2018. doi: 10.1038/s42003-018-0144-1.
Job opportunities
Motivated individuals interested in this work and wishing to do a postdoctoral fellowship or a thesis are encouraged to contact Dr. Lavieu.
Please include your c.v. and a brief research summary, and have three letters of recommendation emailed to Dr. Lavieu.
Team
Julia Dancourt, PhD (Assistant Professor)
Julia is leading the genome-wide screenings effort to identify key actors of EV-delivery.
Sheryl Bui, PhD, (Postdoctoral fellow and MD student)
Sheryl aims at developing EV-mimetics for targeted therapeutics delivery
Pierre Tapie, PhD, Postdoc
Pierre is developing in vitro imaging assay to demonstrate EV fusion in realtime.
Maud Chevé, Engineer,
Maud provides expertise and support in cell biological aspects of the EV-based projects. She is also involved in theproduction of bioengineered EV for thraputical applications.
Shaghayegh Askarian, PhD, Postdoc.
Shaya is characterizing extracellular mitochondria delivery at both cellular and molecular level
Ronald Saraswat, PhD, Postdoc.
Ronald is developing new assay to perform genome wide genetic screening toward EV-content delivery.
Yacine Lahmer, Master student.
Yacine is testing and characterizing protein candidates that act as l negative or positive regulator of EV uptake.
Serge Benichou, PhD (CNRS Research Director)
Serge’s research subgroup recently joined the lab and aims at characterizing HIV spreading through cell-cell fusion.
Alumni
Emeline Bonsergent (PhD student), Zahra El Amir Dache (postdoc), Jihad Karam (postdoc)
À lire aussi
Graduate School Sustainability, Organisations and Institutions Best Thesis Awards: Celebrating Scientific Excellence in Sustainability
The Graduate School Sustainability, Organisations and Institutions held its traditional awards ceremony for the Best Theses of its students. This distinction highlights the scientific excellence of research devoted to sustainable development and sustainability. Meet...

International Day of Women and Girls in Science: celebrating the women who push research forward
February 11 was the International Day of Women and Girls in Science. On this day, Université Paris Cité reaffirms its commitment to the equality between men and women and celebrates the journey of the women who advance research. Between celebrating our heritage and...

Abraha and Pierre: A Friendship to Preserve the People’s Memory of War
In Paris, two historians’ paths crossed. One had just arrived from Ethiopia, carrying notebooks filled with daily observations written during the war in Tigray. The other, based in France, is a specialist of Ethiopian modern history. From this encounter, a partnership...

“Open UE”: looking back on an interdisciplinary adventure organised by the Cardiovascular Sciences Graduate School
The “open UE”, launched by the Graduate School Cardiovascular Sciences, brought together researchers, clinicians, and experts from diverse fields for a week to explore major issues in biomedical and translational research. Open to all students across the 29 Graduate Schools of Université Paris Cité, it offered a unique space for learning and interdisciplinary exchange.
