The Lavieu Lab
In order to help strengthen the international scientific positioning and attractiveness of Université Paris Cité, an action “IdEX Chairs” has been launched. This call for proposals aims to promote the hosting of researchers from outside the institution whose profile corresponds to internationally renowned seniors or juniors with very high potential.
Already 7 laureates in the last two years, including Grégory Lavieu who joined Université Paris Cité on the research site of the Saint-Germain-des-Prés campus to set up a team dedicated to intra- and extracellular membrane transport and thus strengthen the Cell Biology cluster.
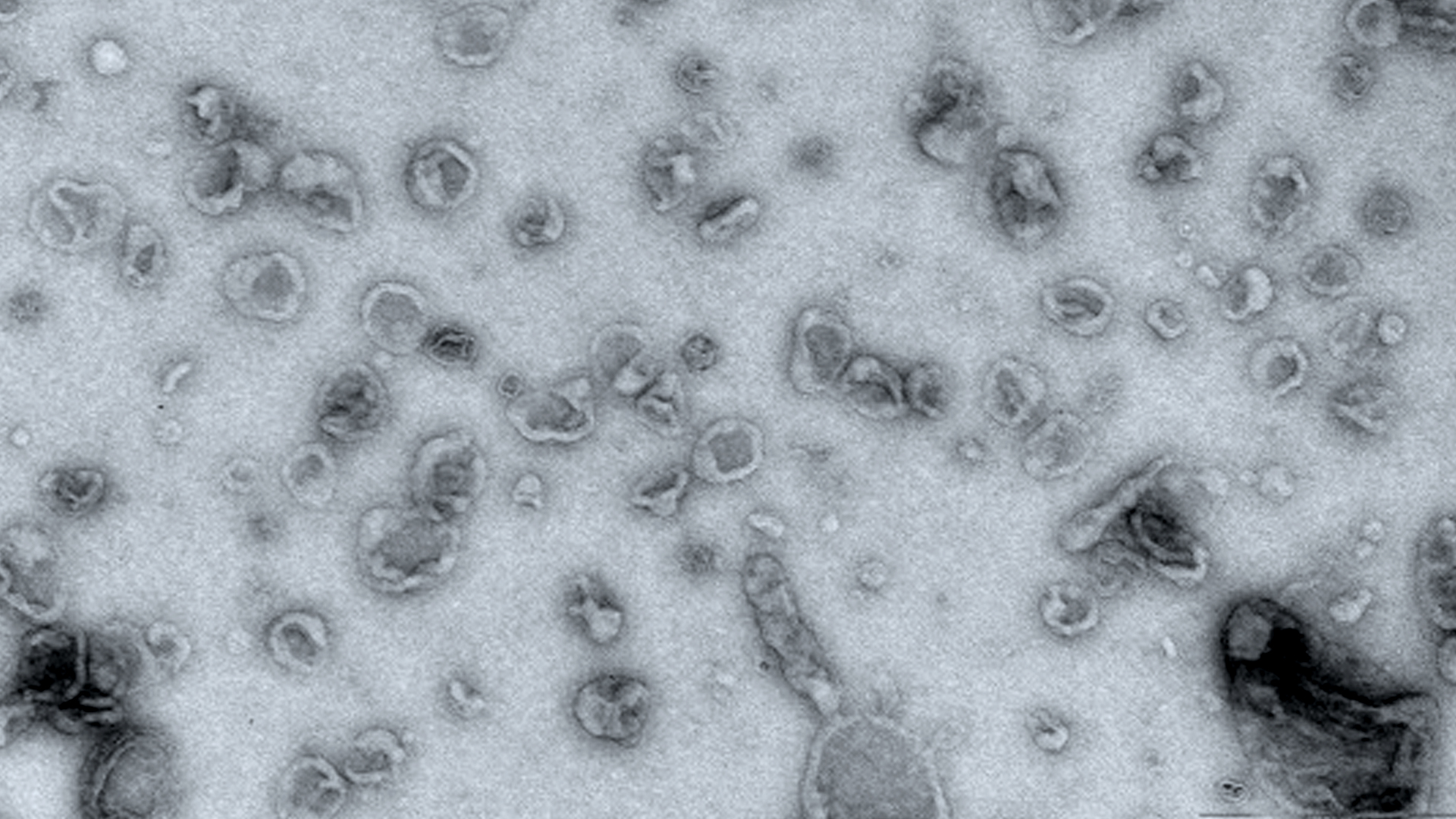
Electron micrograph showing extracellullar vesicles.
Director
 Gregory Lavieu, PhD (PI).
Gregory Lavieu, PhD (PI).
G. Lavieu is a cell biologist/biochemist expert in membrane trafficking. G. Lavieu is currently an INSERM permanent investigator, at the head of the ERL U1316, and “Chaire d’ Excellence” at Université Paris Cité, within the UMR7057 Research Unit.
Previously, he identified and characterized a new mode of intra-Golgi transport, termed Rim progression, dedicated to oversized proteins that are too large to fit into classical transport vesicles.
More recently, G. Lavieu and his research group aim at characterizing the intercellular transport mediated by extracellular vesicles (exosomes), at both cellular and molecular levels. Our goal is to identify the core machinery controlling this process.
Contact
Gregory Lavieu, PhD
Chaire IdEX Université Paris Cité
INSERM ERL U1316 Membranes Dynamics In and Outside the Cell
INSERM, UMR7057/MSC, Bureau P335B
45 rue des Saints-Pères,
75006, Paris, France
Phone : +33 176534267
Gregory.lavieu@inserm.fr
Research:
Extracellular Vesicles (EVs), including Exosomes, are now recognized as vectors of intercellular communication capable of transferring nucleotides, lipids, and proteins from donor to acceptor cells. EV-mediated communication has been associated with many physiological and pathophysiological functions, including cancer, immune responses, cardiovascular diseases, lipid homeostasis, tissues regeneration and stem cell-based therapy.
EVs are being recognized as vectors of major importance for physiology in general, and appears as promising candidates for translational applications such as targeted therapeutics delivery.
However, the mechanisms responsible for EV delivery within the acceptor cells remain unknown at both the cellular and the molecular levels. How do vesicles enter cells? Is it receptor-dependent? How do vesicles deliver their contents within the cytosol of the acceptor cells? Does it require membrane fusion? If yes, what is the nature of the target membrane and the fusion machinery? Those basic questions are not yet answered.
This is not satisfying, especially considering how much we know about the cellular and molecular mechanisms that regulate the delivery of viruses or the transport of intracellular vesicles, which both share several physico-chemical properties with EVs.
It is therefore of high priority to close these gaps, especially when considering the high translational impact of EVs.
We developed cell-free and cell-based assays to accurately assess in a qualitative and quantitative manner the EV delivery process, especially the EV content release. Our results suggest that EV content delivery occurs within the endo-lysosomal compartment, through a process that is pH- and protein-dependent and that seems to involve membrane fusion. We are now aiming at identifying the core machinery that controls this process, to later engineer EV-mimetics designed for targeted therapeutics delivery.
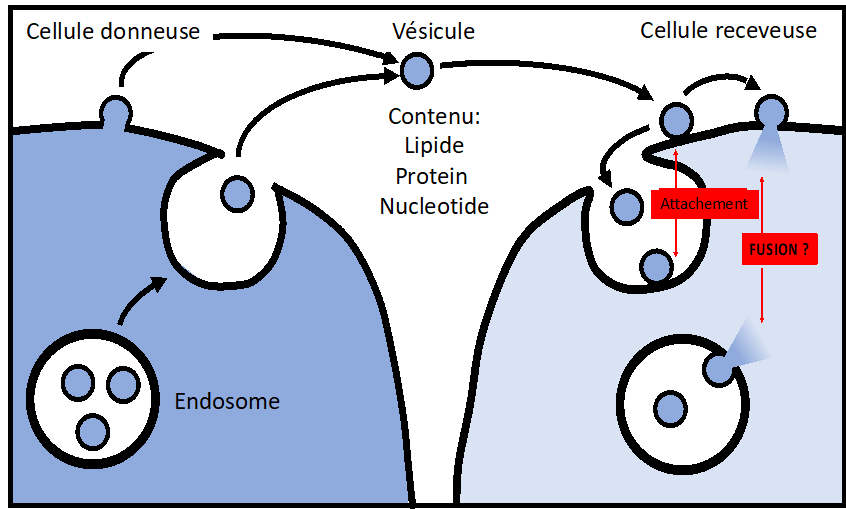
Routes for EV Uptake and Content Delivery
Publications
Selected relevant publications:
- Bonsergent E, Grisard E, Buchrieser J, Schwartz O, Théry C, Lavieu G. Quantitative characterization of extracellular vesicle uptake and content delivery within mammalian cells. Nat Commun. 2021 Mar 25;12(1):1864. doi: 10.1038/s41467-021-22126-y. PMID: 33767144; PMCID: PMC7994380.
- Bonsergent E, Lavieu G. Content release of extracellular vesicles in a cell-free extract. FEBS Lett. 2019 doi: 10.1002/1873-3468.13472.
- Mathieu M, Martin-Jaular L, Lavieu G, Théry C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat Cell Biol. 2019 doi: 10.1038/s41556-018-0250-9. (revue)
- Mocciaro A, Roth TL, Bennett HM, Soumillon M, Shah A, Hiatt J, Chapman K, Marson A, Lavieu G. Light-activated cell identification and sorting (LACIS) for selection of edited clones on a nanofluidic device. Commun Biol. 2018. doi: 10.1038/s42003-018-0034-6.
- Spotlight on early-career researchers: an interview with Gregory Lavieu. Commun Biol. 2018. doi: 10.1038/s42003-018-0144-1.
- Dancourt, J., Piovesana, E. & Lavieu, G. Efficient cell death mediated by bioengineered killer extracellular vesicles. Sci Rep 13, 1086 (2023). https://doi.org/10.1038/s41598-023-28306-8
Job opportunities
Motivated individuals interested in this work and wishing to do a postdoctoral fellowship or a thesis are encouraged to contact Dr. Lavieu.
Please include your c.v. and a brief research summary, and have three letters of recommendation emailed to Dr. Lavieu.
Team
Permanent Teacher/Researcher
Stéphanie Mangenot, PhD Professor, Université Paris Cité
We are studying fusion between EVs and target membranes using in vitro systems.
Researchers
Sheryl Bui MD/PhD student
Sheryl is investigating if viral-derived proteins are involved in EV-delivery and then aims at developing EV-mimetics for targeted therapeutics delivery.
Julia Dancourt, PhD senior scientist
Julia is performing genome-wide screenings to identify key actors of EV-delivery.
Zahra Al Amir Dache, PhD post-doctoral researcher
Zahra is investigating EV-mediated communication between Legionella pneumophila and human target cells, and aims at identifying if the EV-delivery core machinery is conserved through evolution.
Phuong- Ha LE undergraduate student
Ha is testing EV delivery in cells depleted of candidate proteins.
Jihad KARAM post-doctoral Researcher
Jihad is developing novel in vitro assay to image EV membrane fusion.
Buti SURYABRAHMAN post-doctoral Researcher
Buti is characterizing membrane deformation using artificial membranes.
Alumni
Émeline Bonsergent (PhD student), Miranda Bueno (visiting PhD student)
Permanent Investigators (within UMR7057)
Amanda Brun, PhD (CNRS, CRCN) and Florence Gazeau, PhD (CNRS, DR). Applying turbulence on donor cells massively increases EV production. We are investigating the delivery capacity of those turbulence-induced EVs.
À lire aussi
Evidence of magmatically induced faults at the East Pacific Rise
By comparison of ultra-high-resolution 3-D seismic imagery and bathymetry data collected at the East Pacific Rise (EPR) 9º50'N, researchers reveal the existence of magmatic induced faults near the ridge axis.

Convergences: A Strategic Alliance paving the Future of Biology-Health Research
A key milestone for the partnership between Institut Pasteur and Université Paris Cité, the first “Convergences” scientific seminar took place on June 17th at the university’s head office at Odéon.

Université Paris Cité receives 2 grants from Marie Sklodowska Curie Actions programme !
The results of the MSCA Staff Exchange 2023 call for proposals were published on May 28 2024. Université Paris Cité congratulates its two winners, Sylvain Chaty, Professor and Vice President for Culture, Science and Society at the AstroParticle and Cosmology...

MENTOR: Winner of the MSCA Doctoral Networks 2023
When abnormally activated, the mTOR protein can contribute to the development of cancers and certain monogenic diseases that affect around 2 million people worldwide. The multi-disciplinary MENTOR project, which stands for Metabolic control of cell growth by mTOR in...
